A fundamental principle of molecular biology is that structure determines properties which determine biological function. This is what drives massive scientific industry of protein structure determination. An interesting press report led me to read the following paper.
Structural characterization of a novel monotreme-specific protein with antimicrobial activity from the milk of the platypus J. Newman, J. A. Sharp, A. K. Enjapoori, J. Bentley, K. R. Nicholas, T. E. Adams and T. S. Peat
The reason this got my attention was that my late father would have been very interested in the paper. A major research interest of his was milk proteins and he did write several papers on the milk proteins of the echnida and platypus. These fascinating animals are unique to Australia and Papua New Guinea and are the only monotremes (mammals that lay eggs) on the planet. My father collaborated with a biologist, Mervyn Griffiths, who was a colourful character, and was adept at finding and catching the platypus and echidnas in the wild and then milking them.
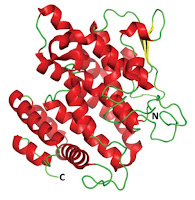
The paper is of scientific interest for two reasons. First, this particular platypus milk protein has anti-bacterial properties. Second, it has a fairly unusual structure, and having a fold that is not found in any other protein. The key question then remains as to whether this unique structural feature is responsible for the biological function.
The presence of the many alpha helices has led the authors to refer to this protein to the Shirley Temple protein in memory of the child movie star's many hair ringlets.
I was happy that an earlier paper about the anti-microbial action by some of the same authors cited many of my fathers papers on monotreme milk proteins, including one paper co-authored with Sir David Phillips. In that paper they tried to deduce the structure of echidna lysozyme and alpha-lactalbumin from similarities to other proteins. Almost 25 years later the platypus paper gives a much more robust determination. This illustrates the expanding success of protein crystallography. In that period the Protein Data Bank has increased from about 1,000 to more than 100,000 protein structures.
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment