In a second class of materials the molecules are not dimerised and the resulting electronic bands are one-quarter filled with holes. Each site in the relevant lattice is a single molecule. The superconductivity emerges out of a charge-ordered [Wigner-Mott] insulator. The simplest possible effective Hamiltonian is an extended Hubbard model at one-quarter filling on a square lattice. In a 2001 PRL Jaime Merino and I showed how superconductivity could occur in these materials as a result of charge fluctuations associated with proximity to charge ordering.
Is this really true? How might you see the charge fluctuations and/or charge order? In crystals where each lattice site is a single atom [e.g. a transition metal ion] one might use inelastic x-ray scattering. However, in molecular systems one has more degrees of freedom since each lattice "site" consists of a large organic molecule. The intramolecular vibrations provide a nice knob to see the local charge density and its fluctuations. Specifically, the frequency and infra-red intensity of an antisymmetric C=C stretch on the BEDT-TTF molecule is particularly sensitive to the charge on the molecule, as parameterised here by Alberto Girlando.
The schematic phase diagram below places two different compounds beta''-M and beta''-SC. The former has a metallic ground state and the latter superconducting and is closer to the charge ordered state.
Bandwidth Tuning Triggers Interplay of Charge Order and Superconductivity in Two-Dimensional Organic Materials
S. Kaiser, M. Dressel, Y. Sun, A. Greco, J.A. Schlueter, G.L. Gard, and N. Drichko
One can contrast the infra-red vibrational spectra of these two compounds. In the lower right of the figure below one sees two sharp vibrational features corresponding to two distinct charge states of the molecule in the beta''-SC compound. At higher temperatures there are large charge fluctuations between these two charge states. In the beta''-M compound one does not see the charge order, just charge fluctuations.
The figure is taken from
Spectroscopic characterization of charge order fluctuations in BEDT-TTF metals and superconductors
A. Girlando, M. Masino, S. Kaiser, Y. Sun, N. Drichko, M. Dressel, H. Mori
There are several interesting issues this work raises and some opportunities for future work.
1. What exactly does the hopping rate [exchange frequency] extracted from the experiment represent physically? How is it (not) related to charge mobility or the diffusion constant associated with charge fluctuations with wave vector (pi,pi)?
2. The theory predicts d_xy superconductivity. This means there should be nodes in the energy gap? are they present? There is some evidence from one penetration depth measurement.
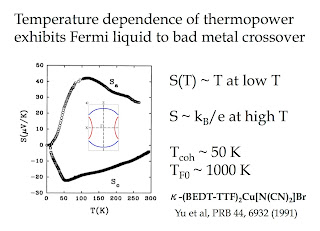
3. Both materials should be bad metals at temperatures of the order of tens of Kelvin. The resistivity is certainly large and a Drude peak is only seen at low temperatures. It would be nice to see some thermopower measurements since they are particularly sensitive to a Fermi liquid bad metal crossover. Theoretical calculations [using the Finite Temperature Lanczos Method] do predict a bad metal close to the charge ordered phase.
4. The title of this post may be an over-simplication. A weak coupling analysis may reveal it is not so easy to separate out spin and charge fluctuations.
I thank Alberto Girlando and Matteo Masino for explaining their work to me.