I watched the movie Moneyball with my family. There was a scene which reminded me of a number of conversations I have had over the years concerning hiring young faculty. The scene features in the trailer below. Billy Beane [Brad Pitt], general manager of the Oakland As major league baseball team is sitting at a table with the club scouts and they are discussing which players to recruit, particularly because they just lost a couple of star players.
The scene highlights how they use arbitrary subjective criteria to endorse or dismiss particular players. ["He has an ugly girlfriend and so must lack self-confidence"!]
How is this like academia?
Unfortunately, the same thing can happen. People can get very emotional and subjective, like in the movie. Over the years, colleagues from a range of institutions have told me how they (or their department) desperately want to hire a particular individual and will "mortgage the farm" to do it.
But, is the individual really worth it? I can remember specific cases over the past 15 years where this has not proven to be the case. In fact, in some cases the individuals concerned are not even doing science any more.
Is the solution using metrics to evaluate potential performance, as in the baseball movie?
No, but it is important to acknowledge that it is fairly difficult to predict future performance. Billy Beane makes that point about himself. He was the high school graduate everyone wanted. But, he flopped in major league baseball.
Friday, September 28, 2012
One minute physics
Maybe you all know all about Minute physics.
But only this week I got introduced (by my wife!) .
We watched Why the Higgs is the missing link and
I think they highlight the value of simple whiteboard talks.
But only this week I got introduced (by my wife!) .
We watched Why the Higgs is the missing link and
I think they highlight the value of simple whiteboard talks.
Labels:
entanglement,
Higgs,
quantum foundations,
talks,
videos
Thursday, September 27, 2012
Geometric distinctions between metals and insulators
Raffaele Resta has a very helpful review article The insulating state of matter: a geometrical theory.
It elegantly connects what to me are a rather disparate range of topics:
Kohn's classic 1964 characterisation of insulators, Berry's phase, Chern numbers, periodic versus open boundary conditions, quantum metrics, Anderson localization, Mott insulators, Quantum Hall insulators, ....
A central quantity defines the "localisation" associated with the ground state wavefunction.
In a 1999 PRL Resta and Sorella introduced this quantity and showed how it diverged at the quantum phase transition between a band insulator and a Mott insulator in a one-dimensional ionic Hubbard model.
In a 2006 J. Chem. Phys. paper Resta connects this localisation to Boy's localisation in quantum chemistry.
It elegantly connects what to me are a rather disparate range of topics:
Kohn's classic 1964 characterisation of insulators, Berry's phase, Chern numbers, periodic versus open boundary conditions, quantum metrics, Anderson localization, Mott insulators, Quantum Hall insulators, ....
A central quantity defines the "localisation" associated with the ground state wavefunction.
In a 1999 PRL Resta and Sorella introduced this quantity and showed how it diverged at the quantum phase transition between a band insulator and a Mott insulator in a one-dimensional ionic Hubbard model.
In a 2006 J. Chem. Phys. paper Resta connects this localisation to Boy's localisation in quantum chemistry.
Wednesday, September 26, 2012
Tailor your poster to your audience
Previously I posted about the importance of tailoring a talk to the audience.
The same is true for posters.
You should not use the same poster for every conference!
The audience may be only theorists, only experimentalists, or a mix, only physicists, only chemists, a mix, only graduate students with diverse backgrounds, only specialists, .....
The background knowledge and the questions that each audience have will be dramatically different.
This post was stimulated by the recent Annual Postgraduate Poster day for the School of Mathematics and Physics at UQ. This is a great initiative as it gives students valuable experience and presenting a poster, and helps everyone get a better idea of what is going on in the School. However, I was struck by how most of the posters I looked at seemed to more appropriate for a specialist conference than for a general audience of physicists and mathematicians. Furthermore, when I asked some of the students to explain their poster to me some quickly resorted to technical jargon and I struggled to put their work in a broader context.
For such a general meeting people have really basic questions like.
What is this field about?
What are the important questions?
How does your work relate to that?
Have you got one simple concrete result that I can understand?
I am not claiming any of the above is easy. But impressing people and connecting with them never is.
The same is true for posters.
You should not use the same poster for every conference!
The audience may be only theorists, only experimentalists, or a mix, only physicists, only chemists, a mix, only graduate students with diverse backgrounds, only specialists, .....
The background knowledge and the questions that each audience have will be dramatically different.
This post was stimulated by the recent Annual Postgraduate Poster day for the School of Mathematics and Physics at UQ. This is a great initiative as it gives students valuable experience and presenting a poster, and helps everyone get a better idea of what is going on in the School. However, I was struck by how most of the posters I looked at seemed to more appropriate for a specialist conference than for a general audience of physicists and mathematicians. Furthermore, when I asked some of the students to explain their poster to me some quickly resorted to technical jargon and I struggled to put their work in a broader context.
For such a general meeting people have really basic questions like.
What is this field about?
What are the important questions?
How does your work relate to that?
Have you got one simple concrete result that I can understand?
I am not claiming any of the above is easy. But impressing people and connecting with them never is.
Tuesday, September 25, 2012
A twisted quantum chemical vision
There is an interesting paper in Science
The Molecular Mechanism of Thermal Noise in Rod Photoreceptors
Samer Gozem, Igor Schapiro, Nicolas Ferré, and Massimo Olivucci
The abstract is quite clear.
If you want a "spherical cow" view of the quantum physics read the beginning of this nice paper by Irene Burghardt and J.T. Hynes.
The Molecular Mechanism of Thermal Noise in Rod Photoreceptors
Samer Gozem, Igor Schapiro, Nicolas Ferré, and Massimo Olivucci
The abstract is quite clear.
Spontaneous electrical signals in the retina's photoreceptors impose a limit on visual sensitivity. Their origin is attributed to a thermal, rather than photochemical, activation of the transduction cascade. Although the mechanism of such a process is under debate, the observation of a relationship between the maximum absorption wavelength (λmax) and the thermal activation kinetic constant (k) of different visual pigments (the Barlow correlation) indicates that the thermal and photochemical activations are related. Here we show that a quantum chemical model of the bovine rod pigment provides a molecular-level understanding of the Barlow correlation. The transition state mediating thermal activation has the same electronic structure as the photoreceptor excited state, thus creating a direct link between λmax and k. Such a link appears to be the manifestation of intrinsic chromophore features associated with the existence of a conical intersection between its ground and excited states.
If you want a "spherical cow" view of the quantum physics read the beginning of this nice paper by Irene Burghardt and J.T. Hynes.
Monday, September 24, 2012
Marketing the Higgs boson
The level of media coverage (and hype) associated with the Higgs boson announcement in July caught me by surprise. But, then I realised that if you spend $10 billion on an experiment there must be some very small fraction associated with publicity and outreach. So I did some Googling and found this CERN document which gives the annual marketing budget as slightly less than $2 million.
Combining this with 10,000 physicists from around the world hitting their local media outlets I should not have been surprised at the level of publicity.
Combining this with 10,000 physicists from around the world hitting their local media outlets I should not have been surprised at the level of publicity.
Friday, September 21, 2012
A helpful sentence to write in any paper
This equation/figure/table is the central result of this paper.
I love to read a sentence such as this. First, it helps me know what the paper is about. Second, it helps me decide whether the paper is important and I should read it in detail. Third, it provides a framework to try and understand the rest of the paper.
Some might complain that their paper contains so many "important" results that it is not possible to identify a single equation or figure which is central. However, I doubt this is true. And if it really is true I would encourage breaking up the paper into several publons.
I love to read a sentence such as this. First, it helps me know what the paper is about. Second, it helps me decide whether the paper is important and I should read it in detail. Third, it provides a framework to try and understand the rest of the paper.
Some might complain that their paper contains so many "important" results that it is not possible to identify a single equation or figure which is central. However, I doubt this is true. And if it really is true I would encourage breaking up the paper into several publons.
Thursday, September 20, 2012
Do you really want to get promoted so soon?
This might seem like a strange idea. Surely everyone wants to get promoted as quickly as possible and to get the associated pay rises?
This post occurs in the context that in some Australian universities it is possible to get promoted through the academic ranks without having tenure. But, even if you have tenure there are reasons why you might not want to get promoted so quickly.
Your primary goal should be to be have a permanent job in a good institution in a place where you and your family want to live. Promotion may make this primary goal harder.
If you don't have tenure and get promoted you may price yourself out of the market of possible permanent jobs or fellowship awards.
In a similar vein, even if you have tenure and hope to make a move to another institution (or country) either for personal or professional reasons, you need to be careful that you also don't price yourself out of the desired market.
For example, suppose you are an Australian living in the UK and you desparately want to return home (understandable!). If you become a Professor in the UK this will significantly limit the routes whereby you can return.
Finally, remember that the more senior you are the more you may be expected to take on more administrative and "leadership" roles. On the flip side, a common "carrot" for people aiming for promotion is to get them to thank on thankless and time-consuming admin tasks that count as "service" in their promotion case. Ask yourself, "Is it worth it?"
In most institutions the best way to get tenure and to get promoted is to get a nice offer from another institution.
This post occurs in the context that in some Australian universities it is possible to get promoted through the academic ranks without having tenure. But, even if you have tenure there are reasons why you might not want to get promoted so quickly.
Your primary goal should be to be have a permanent job in a good institution in a place where you and your family want to live. Promotion may make this primary goal harder.
If you don't have tenure and get promoted you may price yourself out of the market of possible permanent jobs or fellowship awards.
In a similar vein, even if you have tenure and hope to make a move to another institution (or country) either for personal or professional reasons, you need to be careful that you also don't price yourself out of the desired market.
For example, suppose you are an Australian living in the UK and you desparately want to return home (understandable!). If you become a Professor in the UK this will significantly limit the routes whereby you can return.
Finally, remember that the more senior you are the more you may be expected to take on more administrative and "leadership" roles. On the flip side, a common "carrot" for people aiming for promotion is to get them to thank on thankless and time-consuming admin tasks that count as "service" in their promotion case. Ask yourself, "Is it worth it?"
In most institutions the best way to get tenure and to get promoted is to get a nice offer from another institution.
Wednesday, September 19, 2012
Testing the Morse potential
A while back I asked about How good is the Morse potential at describing the potential energy of a chemical bond as a function of stretching of the bond length?
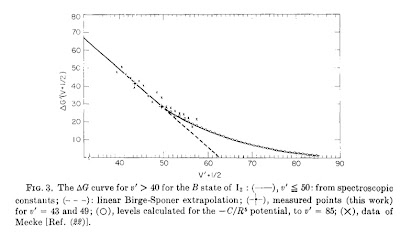
Today I learnt (from Atkins Physical Chemistry text) that Birge-Sponer extrapolation plots provide a means to test the potential all the way up to the disassociation limit. One plots the difference in energy/frequency between neighbouring transitions. If the plot is linear and then where it extrapolates provides a means to determine the disassociation energy.
The Figure above shows a plot for an excited state of the iodine diatomic molecule. The plot is linear all the way up to quantum numbers of order 50. It is taken from this paper.
There is a nice article in Journal of Chemical Education which describes how all of this is (or can be) done in undergraduate Physical Chemistry labs. Pity I missed out...
Today I learnt (from Atkins Physical Chemistry text) that Birge-Sponer extrapolation plots provide a means to test the potential all the way up to the disassociation limit. One plots the difference in energy/frequency between neighbouring transitions. If the plot is linear and then where it extrapolates provides a means to determine the disassociation energy.
The Figure above shows a plot for an excited state of the iodine diatomic molecule. The plot is linear all the way up to quantum numbers of order 50. It is taken from this paper.
There is a nice article in Journal of Chemical Education which describes how all of this is (or can be) done in undergraduate Physical Chemistry labs. Pity I missed out...
Labels:
Born-Oppenheimer,
quantum chemistry,
undergrads
Tuesday, September 18, 2012
Chemistry is quantum science
Owing to its pervasive dependence on the superposition phenomenon, chemistry truly deserves to be called “the quantum science”.This is the concluding sentence of a beautiful article Chemical Bonding as a Superposition Phenomenon by Frank Weinhold in the Journal of Chemical Education.
It is a nice easy introduction to physicists who want to start to get some sense of what chemical bonding is all about.
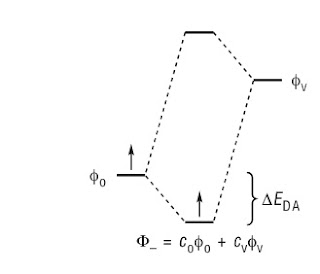
A key idea is that because of the superposition principle that as two chemical units [whether atoms or molecules] are brought closer together, the composite system can lower its energy by being in a superposition state, as illustrated below.
Although the article discusses this idea at the one electron level it is important to realise that this superposition is also relevant for many-body wavefunctions.
The paper also mentions that superposition is at the heart of quantum entanglement, and thus at the heart of quantum weirdness. However, no hints are given of how entanglement may actually play a role in quantum chemistry. That turns out to be a slippery subject, e.g. giving a clear and well-defined measure of entanglement in a simple molecule.
Weinhold's ideas are expanded in great detail in the beautiful book Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective, co-authored with Clark Landis.
Labels:
chemistry,
entanglement,
key concepts,
quantum chemistry
Monday, September 17, 2012
Failure of the spherical cow approximation
Physicists think cows are spherical. But are they?
There is a very nice Perspective in Science Spare the (Elastic) Rod by Phil Nelson. He discusses recent experiments on DNA which show the limitations/breakdown of the much loved [at least by physicists] elastic model rod model for a polymer. This beautifully simple model has only a single parameter, the persistence length, on which length scale it is assumed that the polymer is inflexible. The models shows that the elasticity (on long length scales) of the polymer is due to entropic effects. For DNA the persistence length is estimated to be about one hundred base pairs.
This model is so simple that it can be taught in an undergraduate statistical mechanics class. The mathematics is analogous to a paramagnetic in a magnetic field. Slightly different versions go by names such as Freely Jointed Chain and Worm Like Chain.
A nice review is by Cocco, Marko, and Monasson and contains the helpful figure below.
There is a very nice Perspective in Science Spare the (Elastic) Rod by Phil Nelson. He discusses recent experiments on DNA which show the limitations/breakdown of the much loved [at least by physicists] elastic model rod model for a polymer. This beautifully simple model has only a single parameter, the persistence length, on which length scale it is assumed that the polymer is inflexible. The models shows that the elasticity (on long length scales) of the polymer is due to entropic effects. For DNA the persistence length is estimated to be about one hundred base pairs.
This model is so simple that it can be taught in an undergraduate statistical mechanics class. The mathematics is analogous to a paramagnetic in a magnetic field. Slightly different versions go by names such as Freely Jointed Chain and Worm Like Chain.
A nice review is by Cocco, Marko, and Monasson and contains the helpful figure below.
Friday, September 14, 2012
Two cultures of seminars
Previously I posted about The Two Cultures of C.P. Snow who noted how the academic cultures of science and the humanities diverged in the twentieth century. I agree that both are the poorer for it.
Yesterday I went to a history seminar. The purpose of this post is not to discuss the content, which was fascinating. But rather, to note the striking difference between the mode of presentation and discussion from a typical science seminar.
First, the paper was read. Literally! The speaker had a manuscript which they read without interuption. There were no PowerPoint slides. Yet, the talk/paper was very engaging, interesting, and easy to follow, even for me a non-historian.
Second, the seminar went for 90 minutes, with about 40 minutes devoted to questions and discussions! Probably ten different people asked questions. This was also engaging and interesting. Furthermore, I felt everyone [speaker, questioner, and audience] benefited from this.
One might easily dismiss these differences as being solely due to the different content of disciplines or just how this particular seminar was run. However, I think science seminars and conference presentations could be more interesting and productive if
-they were more accessible
-they had fewer PowerPoint slides (or none!)
-there was more extended time for questions and discussion
-more people asked questions
Yesterday I went to a history seminar. The purpose of this post is not to discuss the content, which was fascinating. But rather, to note the striking difference between the mode of presentation and discussion from a typical science seminar.
First, the paper was read. Literally! The speaker had a manuscript which they read without interuption. There were no PowerPoint slides. Yet, the talk/paper was very engaging, interesting, and easy to follow, even for me a non-historian.
Second, the seminar went for 90 minutes, with about 40 minutes devoted to questions and discussions! Probably ten different people asked questions. This was also engaging and interesting. Furthermore, I felt everyone [speaker, questioner, and audience] benefited from this.
One might easily dismiss these differences as being solely due to the different content of disciplines or just how this particular seminar was run. However, I think science seminars and conference presentations could be more interesting and productive if
-they were more accessible
-they had fewer PowerPoint slides (or none!)
-there was more extended time for questions and discussion
-more people asked questions
Labels:
better science,
C.P. Snow,
history,
philosophy
Thursday, September 13, 2012
Strong electronic correlations in diamond qubits
Yesterday I read a nice paper
Mechanism for optical initialization of spin in NV− center in diamond
by SangKook Choi, Mahish Jain, and Steven Louie
The NV- center in diamond has attracted renewed interest in the past few years because it can be used as a qubit and experimentalists have created entangled states associated with it.
Outstanding questions concern the nature, ordering, and quantum numbers of the low-lying states of a single NV- center. This is key to understanding the process whereby one creates a qubit with a coherence time of order of milliseconds following optical excitation. A previous post discussed this and a recent article by Doherty, Manson, Delaney and Hollenberg provides a helpful review.
The authors obtain definitive results by considering a 4 site Hubbard model with 6 electrons. The model parameters are evaluated from ab initio electronic structure calculations. The 4 sites correspond to orbitals localised on the 3 carbon atoms and one nitrogen atom next to the carbon vacancy.
They find that the Hubbard U is of order 3-4 times the hopping integral and that electronic correlations play a significant role, having a significant effect on the relative energies of the excited states. Previous studies have not treated these correlation effects adequately and so have obtained spurious results. Specifically, the singlet excited states cannot be described in terms of a single Slater determinant.
The effect of correlations is illustrated in the Figure below which shows the energy of the states as a function a structural relaxation [the N atom moves towards the vacancy while the neighbouring C atoms move away]. GEG denotes Ground state Equilibrium Geometry, and EEG denotes Excited state Equilibrium Geometry.
The left panel gives results for exact diagonalisation of the Hubbard model and the right panel for a perturbative treatment based in Density Functional Theory [GW-BSE].
A few minor comments.
1. The authors point out an outstanding question concerns the mechanism whereby the 1A1 excited singlet state decays non-radiatively in 1 nsec to the 1E ground singlet state.
2. Calculating the energy levels for defects in diamond has a long history which seems to have been forgotten. A seminal paper from 1957 was by [my hero] Charles Coulson and Mary Kearsley. They pointed out the utility of a molecular orbital description.
3. Because the nitrogen atomic orbitals are much lower in energy (about 2.6 eV) than the carbon orbitals it should be possible to "integrate them out" and reduce the 4 site 6 electron model to a 3 site 4 electron model where the "carbon" sites have some admixture of the nitrogen orbitals.
4. Using the C3 symmetry of the system it is possible to block diagonalise the Hubbard model into blocks with dimension no larger than 2. The notes below show the energy eigenvalues and quantum numbers for 2 holes on 3 sites with the sign of the hopping integral the same as for the NV- center. The triplet states involve a single Slater determinant. The singlet states all involve two Slater determinants. In the parameter regime relevant to the NV- center, the two determinants have comparable coefficients in all the wavefunctions.
Mechanism for optical initialization of spin in NV− center in diamond
by SangKook Choi, Mahish Jain, and Steven Louie
The NV- center in diamond has attracted renewed interest in the past few years because it can be used as a qubit and experimentalists have created entangled states associated with it.
Outstanding questions concern the nature, ordering, and quantum numbers of the low-lying states of a single NV- center. This is key to understanding the process whereby one creates a qubit with a coherence time of order of milliseconds following optical excitation. A previous post discussed this and a recent article by Doherty, Manson, Delaney and Hollenberg provides a helpful review.
The authors obtain definitive results by considering a 4 site Hubbard model with 6 electrons. The model parameters are evaluated from ab initio electronic structure calculations. The 4 sites correspond to orbitals localised on the 3 carbon atoms and one nitrogen atom next to the carbon vacancy.
They find that the Hubbard U is of order 3-4 times the hopping integral and that electronic correlations play a significant role, having a significant effect on the relative energies of the excited states. Previous studies have not treated these correlation effects adequately and so have obtained spurious results. Specifically, the singlet excited states cannot be described in terms of a single Slater determinant.
The effect of correlations is illustrated in the Figure below which shows the energy of the states as a function a structural relaxation [the N atom moves towards the vacancy while the neighbouring C atoms move away]. GEG denotes Ground state Equilibrium Geometry, and EEG denotes Excited state Equilibrium Geometry.
The left panel gives results for exact diagonalisation of the Hubbard model and the right panel for a perturbative treatment based in Density Functional Theory [GW-BSE].
A few minor comments.
1. The authors point out an outstanding question concerns the mechanism whereby the 1A1 excited singlet state decays non-radiatively in 1 nsec to the 1E ground singlet state.
2. Calculating the energy levels for defects in diamond has a long history which seems to have been forgotten. A seminal paper from 1957 was by [my hero] Charles Coulson and Mary Kearsley. They pointed out the utility of a molecular orbital description.
3. Because the nitrogen atomic orbitals are much lower in energy (about 2.6 eV) than the carbon orbitals it should be possible to "integrate them out" and reduce the 4 site 6 electron model to a 3 site 4 electron model where the "carbon" sites have some admixture of the nitrogen orbitals.
4. Using the C3 symmetry of the system it is possible to block diagonalise the Hubbard model into blocks with dimension no larger than 2. The notes below show the energy eigenvalues and quantum numbers for 2 holes on 3 sites with the sign of the hopping integral the same as for the NV- center. The triplet states involve a single Slater determinant. The singlet states all involve two Slater determinants. In the parameter regime relevant to the NV- center, the two determinants have comparable coefficients in all the wavefunctions.
I thank Taras Plakhotnik for bringing the paper to my attention and reviving my interest in this problem.
Labels:
entanglement,
quantum chemistry,
strong correlations
Wednesday, September 12, 2012
2012 Nobel Prize predictions
People are starting to make predictions. A post from last year links to prediction from other bloggers, mostly in Chemistry.
1. Experiments for testing Bell inequalities and elucidating the role of entanglement in quantum physics
Alan Aspect, John Clauser, and Anton Zeilinger
They received the Wolf Prize in 2010, a common precursor to the Nobel.
2. Duncan Haldane and David Thouless
Showing the important role of topology in low-dimensional condensed matter
This should be a precursor to any prize for topological insulators. I remain to be convinced that there should be a prize for that. Also note Haldane and Thouless both wrote papers that were foundational for topological insulator theory.
I was convinced of the importance of Haldane and Thouless by Rajiv Singh several years ago.
I think their contributions are more original, significant and profound than Berry and Aharonov. But, I think the latter are probably more popular and likely.
Also, Haldane has not received the Wolf Prize yet.
3. Higgs, Kibble, and Englert?
They must be hot candidates and CERN will be lobbying hard. But, I hope it won't happen this year. I would like to see more statistics and checks on the experimental data. Also it has not yet been established that the Higgs field is the origin of the mass of fermions.
I welcome alternative views and suggestions.
1. Experiments for testing Bell inequalities and elucidating the role of entanglement in quantum physics
Alan Aspect, John Clauser, and Anton Zeilinger
They received the Wolf Prize in 2010, a common precursor to the Nobel.
2. Duncan Haldane and David Thouless
Showing the important role of topology in low-dimensional condensed matter
This should be a precursor to any prize for topological insulators. I remain to be convinced that there should be a prize for that. Also note Haldane and Thouless both wrote papers that were foundational for topological insulator theory.
I was convinced of the importance of Haldane and Thouless by Rajiv Singh several years ago.
I think their contributions are more original, significant and profound than Berry and Aharonov. But, I think the latter are probably more popular and likely.
Also, Haldane has not received the Wolf Prize yet.
3. Higgs, Kibble, and Englert?
They must be hot candidates and CERN will be lobbying hard. But, I hope it won't happen this year. I would like to see more statistics and checks on the experimental data. Also it has not yet been established that the Higgs field is the origin of the mass of fermions.
I welcome alternative views and suggestions.
Tuesday, September 11, 2012
Don't believe textbooks
There is a really nice article
What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions
by Frank Weinhold and Roger Klein
It contains the following wonderful paragraph
[Aside: this is essentially the same quantum physics as in my recent paper on H-bonding].
They also give a nice explanation of why simple classical electrostatic calculations sometimes give approximately the right binding energies for the wrong reasons. At the equilibrium geometry the classical electrostatic interaction energy is of the order of a few kcal/mol. These calculations neglect the significant steric repulsion energy (a quantum effect) which in reality is overcome by the strong (quantum) attractive interaction associated with "ionic-covalent resonance".
What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions
by Frank Weinhold and Roger Klein
It contains the following wonderful paragraph
The definitions of H-bonding to be found in current textbooks (or indeed those of the past half-century) employ near-identical verbiage to express concurrence with the classical electrostatic viewpoint, viz. “a special type of dipole–dipole force” 10, “particularly strong dipole–dipole forces” 11, “an extreme form of dipole–dipole interaction” 12, “unique dipole–dipole attractions” 13, “a sort of super dipole–dipole force”14, and the like. Popular molecular mechanics (MM) and molecular dynamics (MD) potentials 15 exhibit similarly unanimous adherence to classical electrostatic functional forms to ‘simulate’ H-bonding, thereby defining how this phenomenon has been represented to MM/MD users since the 1960s. In the face of such unanimity, the title question may seem to have been long since settled!The authors calculate the dipole-dipole interaction for twenty different hydrogen bonded complexes and find there is no correlation (chi less than 0.1!) with the actual binding energies. In contrast there are strong correlations with quantum signatures such as the intermolecular bond order and the "ionic-covalent resonance" character of the ground state wavefunction.
[Aside: this is essentially the same quantum physics as in my recent paper on H-bonding].
They also give a nice explanation of why simple classical electrostatic calculations sometimes give approximately the right binding energies for the wrong reasons. At the equilibrium geometry the classical electrostatic interaction energy is of the order of a few kcal/mol. These calculations neglect the significant steric repulsion energy (a quantum effect) which in reality is overcome by the strong (quantum) attractive interaction associated with "ionic-covalent resonance".
Labels:
books,
chemistry,
hydrogen bonds,
teaching
Monday, September 10, 2012
Face it: some students do cheat!
The New York Times has a good article Studies show more students cheating, with high achievers no exception which is worth reading.
Here are a few practical suggestions to those of us involved in teaching.
1. Don't live in denial. It does happen.
2. Make sure at several stages in a course (e.g., the introduction, before specific assessment is due) tell/remind/exhort students about cheating. Try and be specific about what it is and isn't.
3. Don't assume that just because you said it once in class or wrote it in the course profile that students heard, understood, or internalised it.
4. When it does happens, make sure you do discipline students, in accordance with course and institutional policies. Private words and "slaps on the wrist" are ineffective. It is very important that student records reflect repeat offences.
Unfortunately, all of the above is a hassle and just increases our workload. But, if we don't act nothing is going to change.
I welcome other suggestions.
Here are a few practical suggestions to those of us involved in teaching.
1. Don't live in denial. It does happen.
2. Make sure at several stages in a course (e.g., the introduction, before specific assessment is due) tell/remind/exhort students about cheating. Try and be specific about what it is and isn't.
3. Don't assume that just because you said it once in class or wrote it in the course profile that students heard, understood, or internalised it.
4. When it does happens, make sure you do discipline students, in accordance with course and institutional policies. Private words and "slaps on the wrist" are ineffective. It is very important that student records reflect repeat offences.
Unfortunately, all of the above is a hassle and just increases our workload. But, if we don't act nothing is going to change.
I welcome other suggestions.
Saturday, September 8, 2012
Is this test of the Standard Model impressive?
A week ago we had an interesting physics colloquium The Higgs Boson at the Large Hadron Collider by Elizabetta Barberio, who works in the ATLAS detector collaboration.
It was nice to hear a talk about the Higgs boson which simply discussed the physics and the actual experimental results, without any hype.
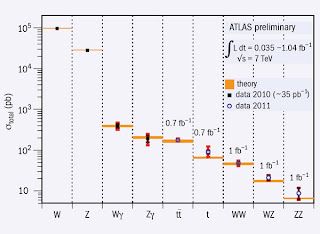
To me one of the most interesting and impressive figures shown in the talk actually had nothing to do with the Higgs! I found it here on the CERN website.
It shows the measured cross sections for the production of different particle products (horizontal scale).
Note the vertical scale varies by four orders of magnitude.
Basically, it shows that the results from the ATLAS detector are consistent with the Standard Model.
One thing I would like to know is how many independent parameters (coupling constants) are involved in determining these cross sections from the Standard Model?
Is the agreement between experiment and theory impressive? Or are there so many parameters in the Standard Model that this plot is really just saying what those parameters are? But, all the cross sections can't be independent of one another.
It was nice to hear a talk about the Higgs boson which simply discussed the physics and the actual experimental results, without any hype.
To me one of the most interesting and impressive figures shown in the talk actually had nothing to do with the Higgs! I found it here on the CERN website.
It shows the measured cross sections for the production of different particle products (horizontal scale).
Note the vertical scale varies by four orders of magnitude.
Basically, it shows that the results from the ATLAS detector are consistent with the Standard Model.
One thing I would like to know is how many independent parameters (coupling constants) are involved in determining these cross sections from the Standard Model?
Is the agreement between experiment and theory impressive? Or are there so many parameters in the Standard Model that this plot is really just saying what those parameters are? But, all the cross sections can't be independent of one another.
Friday, September 7, 2012
Cake meeting talk on thermopower
This week I talk about Kelvin formula for the thermopower. Here are the slides. Not sure that they will be much use for people not at the meeting. At least, they give a flavour of some of the experimental results of particular interest to me. Most have been featured in earlier blog posts on the subject.
I made one small discovery in my preparation: a specific example where the Kelvin formula is exact. If one takes the temperature dependence of the chemical potential for non-interacting fermions with a general density of states [equation 2.77 in Ashcroft and Mermin] then the thermopower from the Kelvin formula is exactly that given by the Mott-Heikes formula obtained from solving the Boltzmann equation [equation 13.62 in Ashcroft and Mermin] for the case where the scattering time and velocity have no energy dependence.
I made one small discovery in my preparation: a specific example where the Kelvin formula is exact. If one takes the temperature dependence of the chemical potential for non-interacting fermions with a general density of states [equation 2.77 in Ashcroft and Mermin] then the thermopower from the Kelvin formula is exactly that given by the Mott-Heikes formula obtained from solving the Boltzmann equation [equation 13.62 in Ashcroft and Mermin] for the case where the scattering time and velocity have no energy dependence.
Labels:
Fermi liquids,
strong correlations,
talks,
thermoelectric
A systematic approach to career planning
Face it: most science Ph.Ds will end up working outside academia. What are the options?
There is a really interesting Editorial in Science this week Planning Career Paths for Ph.Ds. Here is part of it describing Individual Development Plans (IDP)
This sounds like a great initiative.
I look forward to hearing from people who have used this new web resource.
There is a really interesting Editorial in Science this week Planning Career Paths for Ph.Ds. Here is part of it describing Individual Development Plans (IDP)
A free Web application for this purpose, called myIDP, has become available this week. It is designed to guide early-career scientists through a confidential, rigorous process of introspection to create a customized career plan. Guided by expert knowledge from a panel of science-focused career advisers, each trainee's self-assessment is used to rank a set of career trajectories. After the user has identified a long-term career goal, myIDP walks her or him through the process of setting short-term goals durected toward accumulating new skills and experiences important for that career choice. After each step, the user updates the plan, documenting efforts and progress. The user can opt to receive monthly e-mail reminders from myIDP to stay focused on goals and update progress and plans. Very importantly, the plan can be altered as skills develop, interests change, and career objectives are reconsidered.
This sounds like a great initiative.
I look forward to hearing from people who have used this new web resource.
Thursday, September 6, 2012
A limit to my understanding
One of the many things I find hard to understand about quantum many-body theory is the fact that the order in which you take the limits of functions really does matter. I fear I was brainwashed by too many calculus classes which always considered well defined analytical functions!
Two significant and profound examples concern the theory of superconductivity and the the thermoelectric effect.
Example one: electromagnetic response of a superconductor
Consider the frequency and wavevector dependence of the current-current correlation function, denoted Lambda(q,omega) below, where q is the wavevector and omega the frequency. The q to 0 limit can also be viewed as taking the thermodynamic limit.
The following equations are taken from an important 1993 paper Insulator, metal, or superconductor: the criteria by Doug Scalapino, Steve White, and Shoucheng Zhang
Example two: thermoelectric response
A very elegant 2010 paper Kelvin formula for thermopower, Michael Peterson and Sriram Shastry considered the significance of taking limits in different orders. They started with the exact Kubo expression for the frequency and wavevector dependent thermoelectric response S(q,omega).
The correct value for the Seebeck coefficient is obtained by first taking the q to 0 (i.e. thermodynamic limit of infinite system size) and then taking the static (omega to 0) limit. However, if one reverses the order of these two limits S(q,omega) reduces to Kelvin's (1854) formula which gives the Seebeck coefficient (n.b. a transport property) in terms of purely thermodynamical variables, i.e. S is proportional to the derivative of the chemical potential with respect to temperature. This formula is only approximate, but is very useful particularly for getting magnitudes and trends.
The slide below from a 2012 talk by Shastry nicely summarises the above.
Can someone give other examples?
Two significant and profound examples concern the theory of superconductivity and the the thermoelectric effect.
Example one: electromagnetic response of a superconductor
Consider the frequency and wavevector dependence of the current-current correlation function, denoted Lambda(q,omega) below, where q is the wavevector and omega the frequency. The q to 0 limit can also be viewed as taking the thermodynamic limit.
The following equations are taken from an important 1993 paper Insulator, metal, or superconductor: the criteria by Doug Scalapino, Steve White, and Shoucheng Zhang
The first equation defines the superfluid density D_s, and the last one the Drude weight D. The two quantities are equal in a BCS superconductor but are not equal in a non-superconducting metal or in unconventional superconductors such as cuprates or organic charge transfer salts.
The second equation is required by gauge invariance. Comparing to the first equation we see that one gets a different answer depending on the order in which q_x (parallel to the current) and q_y goes to zero.
Example two: thermoelectric response
A very elegant 2010 paper Kelvin formula for thermopower, Michael Peterson and Sriram Shastry considered the significance of taking limits in different orders. They started with the exact Kubo expression for the frequency and wavevector dependent thermoelectric response S(q,omega).
The correct value for the Seebeck coefficient is obtained by first taking the q to 0 (i.e. thermodynamic limit of infinite system size) and then taking the static (omega to 0) limit. However, if one reverses the order of these two limits S(q,omega) reduces to Kelvin's (1854) formula which gives the Seebeck coefficient (n.b. a transport property) in terms of purely thermodynamical variables, i.e. S is proportional to the derivative of the chemical potential with respect to temperature. This formula is only approximate, but is very useful particularly for getting magnitudes and trends.
The slide below from a 2012 talk by Shastry nicely summarises the above.
Can someone give other examples?
Wednesday, September 5, 2012
RVB history and citations
In a talk he gave for a UC Davis Physics Colloquium in 2008 Sriram Shastry describes the background to his discovery of the Shastry-Sutherland lattice model [published in Physica B!] and spinons in the Majumdar-Ghosh model. [He received the 2009 Onsager Prize from the American Physical Society for this work].
Anderson (1973) Cambridge preprint!!It is worth noting that this 1973 paper now has more than 500 citations (in Scopus) and was the key idea behind Anderson's 1987 Science paper (5000+ citations). This is another example that citations on a 5 year time scale can be quite misleading about the significance of a piece of work.
Revived Pauling’s idea on resonance and gave a rather poor energy gs for Heisenberg antiferromagnet on the triangular lattice:
He did not fool us with the poor numbers emerging from his calculation: We were thoroughly impressed in 1973 by the originality and depth of his ideas!!
However, we were in a minority since there are only 5 or 6 other papers between 1973 and 1986 citing Anderson RVB, and 3 of them are mine and 2 by Anderson!!
Labels:
journals,
P.W. Anderson,
spin liquid,
valence bond theory
Tuesday, September 4, 2012
Signatures of "band-like" transport in organic electronic materials
I used to regularly write posts about charge transport in organic electronic materials. Some of these generated lively discussion in the comments section.
This morning I read an interesting paper Band-Like Electron Transport in Organic Transistors and Implication of the Molecular Structure for Performance Optimization
by Nikolas Minder, Shimpei Ono, Zhihua Chen, Antonio Facchetti, Alberto Morpurgo
They correctly distinguish "band-like" transport where a charge carrier is delocalised over just a few molecules from true band transport where it is delocalised over a large number of molecules [or unit cells in a crystalline semiconductor such as silicon].
They claim that a signature of band-like transport is the common observation of a mobility that decreases with increasing temperature and a Hall effect signal. I agree with the former but am confused about the latter. I thought for incoherent polaron transport one could still have a Hall effect, as discussed in a classic paper by Friedman and Holstein.
The authors overlook the fact that a signature of band-like transport is that the mobility should be larger than e a^2/hbar ~ 1 cm^2/Vsec. Ignorance of this old and important result seems to be common in the field.
Previously, I pointed out that comparing the relative magnitudes of the energy gaps respectively associated with mobility, optical conductivity, and thermopower is a nice way to distinguish coherent from incoherent transport.
This morning I read an interesting paper Band-Like Electron Transport in Organic Transistors and Implication of the Molecular Structure for Performance Optimization
by Nikolas Minder, Shimpei Ono, Zhihua Chen, Antonio Facchetti, Alberto Morpurgo
They correctly distinguish "band-like" transport where a charge carrier is delocalised over just a few molecules from true band transport where it is delocalised over a large number of molecules [or unit cells in a crystalline semiconductor such as silicon].
They claim that a signature of band-like transport is the common observation of a mobility that decreases with increasing temperature and a Hall effect signal. I agree with the former but am confused about the latter. I thought for incoherent polaron transport one could still have a Hall effect, as discussed in a classic paper by Friedman and Holstein.
The authors overlook the fact that a signature of band-like transport is that the mobility should be larger than e a^2/hbar ~ 1 cm^2/Vsec. Ignorance of this old and important result seems to be common in the field.
Previously, I pointed out that comparing the relative magnitudes of the energy gaps respectively associated with mobility, optical conductivity, and thermopower is a nice way to distinguish coherent from incoherent transport.
Monday, September 3, 2012
UQ quantum seminar on hydrogen bonding
Here is the current version of my slides for my seminar tomorrow A physicist looks at hydrogen bonding. I have included a fair bit of introductory and background material to help build context and interest. Hopefully I have tailored the talk to the audience.
Sunday, September 2, 2012
Practice makes ... competent
Giving decent talks and writing readable papers is a hard task, even for experienced scientists. How do young inexperienced scientists (especially students and new postdocs) acquire these skills? They are crucial to survival in science.
A key component is practice... practice ... practice, especially when coupled with constructive feedback, from both peers and mentors.
Students and postdocs should take every opportunity they can to give talks whether at group meetings, departmental seminars, or conferences. Giving a practice to a smaller invited friendly audience can be very helpful. Even giving a practice to family members or friends who know nothing about the relevant science can build confidence and smooth the presentation.
Supervisors have a responsibility to provide regular opportunities for students and give constructive feedback. However, if they do not, students should not wait around but create their own private forums and at least give talks to each other.
Similar considerations apply to learning to write papers. Lots of practice is key. Students and postdocs should always write the first draft. This may be not the quickest and most efficient way to produce a paper. But, it is a necessary ingredient in training.
A key component is practice... practice ... practice, especially when coupled with constructive feedback, from both peers and mentors.
Students and postdocs should take every opportunity they can to give talks whether at group meetings, departmental seminars, or conferences. Giving a practice to a smaller invited friendly audience can be very helpful. Even giving a practice to family members or friends who know nothing about the relevant science can build confidence and smooth the presentation.
Supervisors have a responsibility to provide regular opportunities for students and give constructive feedback. However, if they do not, students should not wait around but create their own private forums and at least give talks to each other.
Similar considerations apply to learning to write papers. Lots of practice is key. Students and postdocs should always write the first draft. This may be not the quickest and most efficient way to produce a paper. But, it is a necessary ingredient in training.
Saturday, September 1, 2012
Why does liquid water have a relatively high boiling point?
The graph below shows the boiling points of different molecular liquids of the chemical composition HnX. Note that at room temperature all of them, except H2O would be vapour.
What is so different about water?It can form much stronger hydrogen bonds than the heavier ones, because X=O is the most electronegative.
H2O has two proton donors and two lone pair orbitals which act as acceptors for hydrogen bonds. Hence, each molecule can form four hydrogen bonds in a tetrahedrally co-ordinated liquid.
In contrast NH3 has three donors and one acceptor. Hence, it can only form two hydrogen bonds with neighbours.
This can all be illustrated with some very cute pictures of little men grabbing hands and legs in Philip Ball's book H2O: a biography of water.
I first saw all this nicely explained by Jim Skinner a few months ago in a Town Talk at the Telluride Science Research Center in Colorado.
Subscribe to:
Posts (Atom)